著者:Marcel Besier, Senior Director, Services Delivery, EMEA
今日 ますます複雑化する 状況において臨床試験を管理することは非常に難しく、患者の同意取得、薬剤の提供、アウトカムデータの収集を円滑に進めるためには、しばしば複数のプロセスを調整する必要があります。第 III 相臨床試験では、1試験当たり平均 360 万のデータポイント が生成されており(過去10 年間で 3 倍に増加)、治験実施施設チームにかかる負担は前例のないほど大きくなっています。試験テクノロジーのポートフォリオが拡大し、研究の支援と迅速化が進むにつれ、すでに過度な負担を強いられている治験実施施設チームに、意図せず新たな負担が生じる可能性があります。
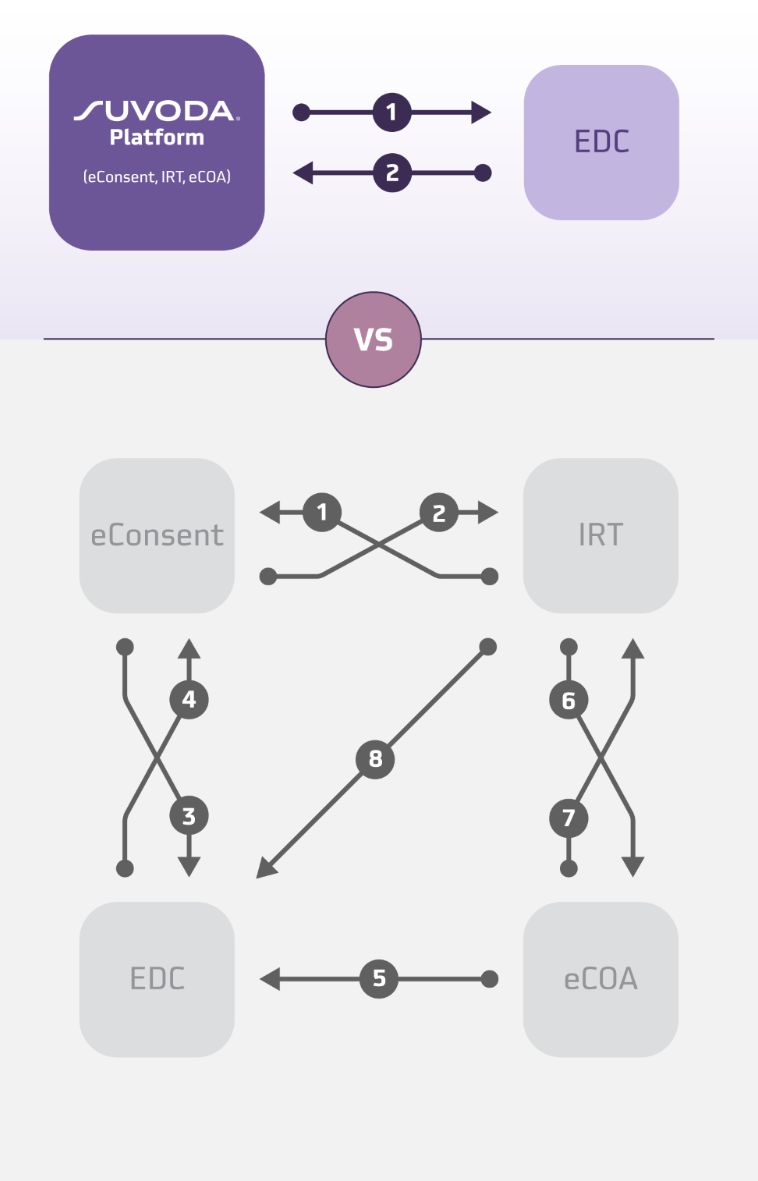
この状況において、統合テクノロジープラットフォームは、eConsent から IRT、eCOA までの重要なツールを単一のインターフェースにシームレスに統合することで、治験実施施設と治験依頼者にとって非常に有益なツールとなります。 試験管理を簡素化し、統合作業を削減することは、治験実施施設チームと治験依頼者にとって非常に重要な要素です。
eConsent、IRT、eCOA を統合した患者管理プラットフォームにより、特に複雑な試験において、治験実施施設と治験依頼者の業務を簡素化できます。
- リアルタイムでの同意取得、データおよび在庫の監視と管理: リアルタイムでの同意取得、データおよび在庫の監視と管理: リアルタイムな患者データ活用により、薬剤供給の合理化や在庫管理の精度向上、供給予測の改善が可能となり、臨床医が適切な薬剤を投与できるよう支援します
- コンプライアンスの強化と正確な薬剤交付: eConsent を IRT と統合することにより、仮想の「ゲート」として機能します。 最新の同意を得た患者さんのみが薬剤を受け取ることになるため、許可なく薬剤が交付されるリスクが大幅に減少します。さらに、システム間でデータをリアルタイムで監視できるため、潜在的な問題が発生した際に警告を出し、コンプライアンスの維持を積極的にサポートします。
- シームレスなデータ共有: 患者中心の統合 eClinical エコシステムにより、データが自動的に共有され、システム間での手動データ転送の必要性が減少します。 この方法により、各テクノロジーシステム間のデータを正確に統合し、データの整合性と一貫性を強化できます。
SuvodaのeConsent、IRT、eCOAは、単一のソフトウェアプラットフォーム上でシームレスに連携し、試験チームが業務を効率的に管理できる中央司令センターとして機能します。 加えて、当社は独自のテクノロジー、治験実施計画書及び疾患領域に関する専門知識、さらに単一プロジェクトチームによるサービスモデルを駆使し、治験依頼者および治験実施施設と協力して、不確実性や変更、試験途中での改訂にも効果的に対応します。 このサービス中心の技術アプローチにより、治験実施施設と治験依頼者は使いやすいワークフローを構築し、臨床試験全体を通じて安全なデータ共有が可能になります。これにより、テクノロジーに費やす時間を減らし、命を救う可能性のある治療法の研究により多くの時間を割くことができます。
著者

Marcel Besier
Senior Director, Services Delivery, EMEA
Suvoda
