Improving efficiency in clinical supplies management
In clinical trials, it is critical to ensure that each patient receives the correct medication at the right time and place.
IRT is an important solution to help sponsors manage drug supply efficiently. Although IRT solutions have been part of the clinical trial landscape for some time, their use in clinical trials is still growing.1
Supply chain optimization, reduced drug waste, cost savings, and supporting reduction of the carbon footprint are important benefits of IRT solutions, as supply costs
are significant.
One McKinsey study found that IRT solutions can optimize clinical trial supply chains to reduce waste and associated costs by an estimated 15-20%.2 This can be a critical
tool to meet industry needs, as 94% of life sciences executives said improving supply chain was a top priority in 2021.3
As an example, the median waste level for investigational medicinal product (IMP) kits in the above McKinsey study was at 50%,4 a clear opportunity for improvement. A recent Suvoda webinar poll bolsters these findings; 75% of participants named costs of manufacturing investigational products and sourcing costs for comparators, standard of care, and adjuvant therapies as the major cost drivers in their clinical trial supply.5
Major cost drivers in clinical supply
Webinar participants were asked: What are the biggest clinical supply costs in your trial?

Source: Suvoda. “Major Cost Drivers of Clinical Supply.” October, 2023.
Problems in clinical supply chains create challenges for patients, sites, and sponsors. A LinkedIn poll showed that the majority of the Suvoda community consider missed patient visits to be the greatest challenge for clinical trials when supply is disrupted. As one respondent said, “Missed patient visits = missed data points.”
Implications for clinical trials when supply is disrupted
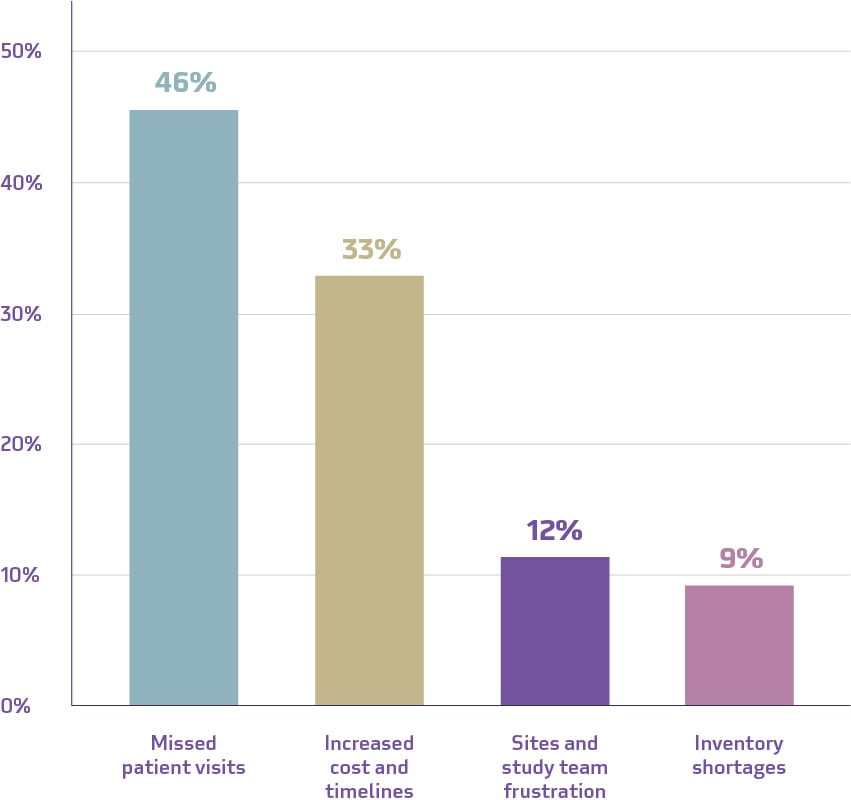
Source: Suvoda. “What are the greatest challenges for trials when clinical supply is disrupted?” March, 2024.
IRT systems are an important tool to address challenges of drug supply, reducing drug waste, and driving cost efficiency. Optimized supply chains help patients, giving the best chance for drugs to reach patients on time.
 FREE EBOOK
FREE EBOOK
Get the full Decoding eClinical Trends eBook
Resources
1“Interactive Response Technology Marketa Size, Share, Growth, and Industry Analysis, by Type (EDC System, CTMS, ECOA Systems), by Application (It, Medical, Drug Control & Others), Regional Insights and Forecast to 2031,” Business Research Insights, December 2023, https://www.businessresearchinsights.com/market-reports/ interactive-response-technology-market-109514
2 Munaf Kachwala et al., “Clinical Supply Chains: How to Boost Excellence and Innovation,” McKinsey & Company, November 29, 2021, https://www.mckinsey.com/ industries/life-sciences/our-insights/clinical-supply-chains-how-to-boost-excellence-and-innovation
3 PricewaterhouseCoopers, “How Health Organizations Can Integrate ESG Priorities,” PwC, accessed March 19, 2024, https://www.pwc.com/us/en/industries/health- industries/library/esg-health-industry.html
4 Munaf Kachwala et al., “Clinical Supply Chains: How to Boost Excellence and Innovation,” McKinsey & Company, November 29, 2021, https://www.mckinsey.com/ industries/life-sciences/our-insights/clinical-supply-chains-how-to-boost-excellence-and-innovation
5 “Major Cost Drivers of Clinical Supply,” Suvoda, October 2023, https://www.suvoda.com/insights/blog/major-cost-drivers-of-clinical-supply
