Snapshot:
- Clearer Patient Understanding Drives Retention: Electronic Informed Consent processes can improve patient understanding of clinical trials, reducing confusion and dropout rates.
- Streamlined Consent Processes Boost Trial Efficiency: eConsent streamlines workflows by reducing paper use, improving data accuracy, and facilitating remote trial participation, leading to cost and time savings.
- Overcoming Barriers to eConsent Adoption: Concerns about patient usability, system integration, and budget constraints continue to hinder widespread eConsent adoption.
Informed consent is the cornerstone of clinical trials, ensuring patients comprehend the specifics and implications of their trial participation. Traditional consent forms, often comprised of highly technical content, may hinder patient understanding and potentially lead to premature trial exit.
Digitizing consent can support better patient comprehension and engagement. Cohen et al report in the Journal of Medical Internet Research that eConsent can offer a more accessible and user-friendly option for patients, with higher satisfaction compared to paper-based methods.1
Notably, many patients, sites, and sponsors appear ready to use eConsent. In a survey of the general population, Suvoda found that overall, a large majority of people in the US and the UK are comfortable signing documents electronically. Half of US respondents indicated that they prefer electronic signature over paper for medical consent forms, as did a significant minority of UK respondents.2
Patient comfort and preference with electronic signatures

Source: Suvoda. “Survey From Suvoda Shows Comfort with Electronic Signatures Is on the Rise, Yet Healthcare Consent Still Lags.” April, 2023.
In another Suvoda eConsent survey of sites and sponsors, we found that although a lack of eConsent adoption exists across sites and sponsors, many do see benefits to its use.
Percentage of sites and sponsors using eConsent

Source: Suvoda. “eConsent Market Survey” October, 2023.
Perceived benefits of eConsent among sponsors and sites
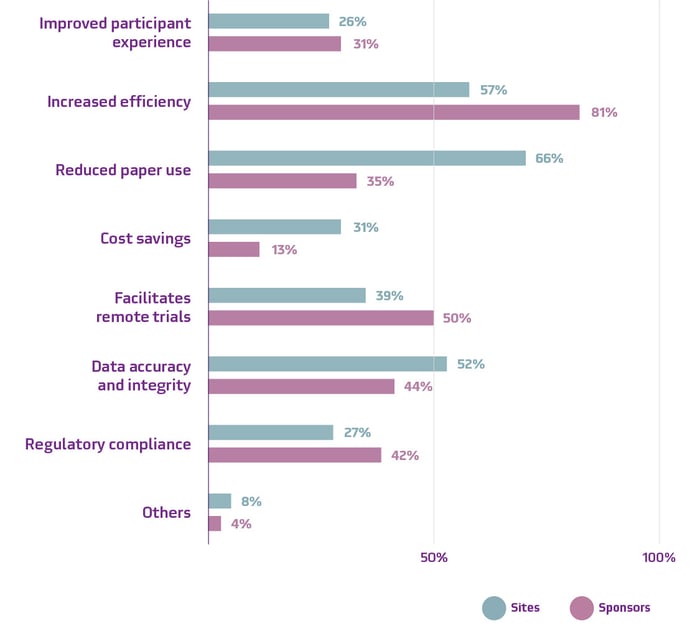
Source: Suvoda. “eConsent Market Survey” October, 2023.
Barriers to eConsent adoption vary. Many sponsors and sites are concerned about usability issues for patients themselves, despite patients’ digital literacy and comfort with electronic signatures.
Perceived barriers to eConsent among sponsors and sites

Percentage of site and sponsor respondents who selected each characteristic as a top 3 concern of adopting an eConsent solution for clinical trials.
Source: Suvoda. “eConsent Market Survey” October, 2023.
An important goal of eConsent is to demystify the consent process in clinical trials, and with near universal tech literacy and increasingly user-friendly tools, it is poised to fulfill that promise. By streamlining consent forms and using clear explanations, eConsent may provide an important step towards enhancing patient comprehension, fostering a more inclusive and patient-centric future in clinical research.
 FREE EBOOK
FREE EBOOK
Get the full Decoding eClinical Trends eBook
Resources
- Edwin Cohen et al., “Comparative Effectiveness of Econsent: Systematic Review,” Journal of Medical Internet Research 25 (September 1, 2023), https://doi. org/10.2196/43883
- “Survey from Suvoda Shows Comfort with Electronic Signatures Is on the Rise, yet Healthcare Consent Still Lags,” Suvoda, April, 2023, https://www.suvoda.com/ insights/all-news/survey-shows-comfort-with-electronic-signatures
