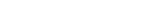A single clinical trial technology platform
Purpose-built
products across the patient journey
Seamless experience
system built from the ground-up
Ease of use
patient-centric workflow
Seamlessly manage mission-critical, time-sensitive, and deeply-human interactions with the Suvoda Platform—a patented ecosystem delivering eConsent, IRT, eCOA, and ePatient.
 In today’s increasingly complex clinical trials, sponsors, sites, and patients need systems that work together seamlessly, integrate easily with other tools, and are efficiently deployed.
In today’s increasingly complex clinical trials, sponsors, sites, and patients need systems that work together seamlessly, integrate easily with other tools, and are efficiently deployed.
With the Suvoda Platform, we bring together all our software products—and their associated data streams—in a purpose-built and dynamic ecosystem that maximizes control during the most urgent clinical trial moments. The result? The right user is thoughtfully engaged, at the right moment, with the information and insights they need to calmly take the right next step. So sponsors, sites, and patients alike feel empowered to take command over the most urgent moments in their journey through a trial.
Our platform delivers:
- Seamless user experience across eConsent, IRT, eCOA, and ePatient
- A single, robust data model for improved data workflow and reduced integrations
- Customer trial standardization and updates without disruption
- Rapid deployment, even for trials with high levels of protocol, operational, logistical, technical, or cultural complexity
Comprehension and control for informed consent
High-quality data from a calm eCOA experience
Simplify patient participation
Four clinical trial products on one platform minimize friction and maximize engagement for sponsors, CROs, sites, and patients.
Suvoda products—eConsent, IRT, eCOA, and ePatient—operate on a single patented software platform with a single, robust data model. That means products are fully integrated from the outset. The Suvoda Platform ensures that interactions and data from one product can automatically direct and drive sponsors, sites, and patients to take required actions in another. Because it’s our goal to not just simplify, but actually enhance the experience and contributions of all users across the clinical trial journey.
Platform features:
- Fully integrated product suite with shared user interface across eConsent, IRT, eCOA, and ePatient
- Single sign on
- Automatic gatekeeping or triggering of activities from one product to another
- User workflow moves smoothly from one tool to the next, without having to switch systems
Patented software design reduces and simplifies integrations, creating a more cohesive eClinical ecosystem.
The Suvoda Platform uses best practice software design approaches, recognized with a US patent, to eliminate integration across our four products—and the comprehensive API surface simplifies integrations with other third-party solutions and sponsor systems. Central data management gives you real-time access to information and confidence that your clinical trial data is secure. One system to let you focus on your trial.
Platform features:
- API surface allows seamless integration with your in-house systems and other third-party clinical technologies
- A single, central, and synchronous patient, drug, and site data model across eConsent, IRT, eCOA, and ePatient
Elegant software architecture means your clinical trial technology will always be current.
Suvoda protects and leverages your eClinical investment with a platform designed to remember your standards and keep your trials up-to-date throughout the study. That means your trial software is built on what you have done before and can be upgraded to to the latest trial technology and functionality. Another way to think about it? Future-proofed eClinical programs.
Platform features:
- Flexible architecture makes mid-study changes and protocol amendments less disruptive
- Options to upgrade your system (or opt-out, if you prefer) when updates become available, without losing any customizations
- The patented Virtual Partition architecture enables advanced customization and stores enterprise standards to easily apply to future trials
Simplify software set up with streamlined deployment, a single project team, and low-code/no-code technology.
Clinical trials today are longer than ever and every day counts. Your single Suvoda project team and streamlined deployment across all four products simplifies your overall implementation. The platform allows you to deploy trial technologies more efficiently and accurately—with low-code/no-code and advanced design tools to power efficient build, deployment, and modification timelines. Even for trials facing high levels of protocol, operational, logistical, technical, or cultural complexities.
Platform features:
- Harmonized deployment process for all four solutions saves study teams time and effort
- Suvoda XD–a proprietary low-code/no-code toolset–streamlines start-up and mid-study changes and improves service delivery
- The Virtual Partition makes customizations and standards easy to implement, for more control over increasing complexity
Elegant user experience, efficient deployment, customizations, and data integrity.
Suvoda’s clinical trial software architecture leverages the best of B2B and consumer technology platforms, and our unique architectural approach was recognized with a US patent. It delivers the intuitive user experience and efficient set up that people expect out of their technology, while providing the data integrity, customizations, and flexibility needed for clinical trials.
Three essential elements make up the platform architecture:
- The Foundation: building on cloud technology best practices
The Suvoda platform includes a comprehensive API-surface, using the latest cloud technologies. - The Virtual Partition: our patented approach to customizable and upgradable software
A Virtual Partition is built into the software architecture, to allow for trial-specific adjustments outside of the core product code. It allows customization, with benefits of SaaS: upgradability, cloud-based deployment, and a shared data layer. - Elegant user experience, efficient deployment, customizations, and data integrity.
The tools allow our teams to:
-
-
- Leverage Suvoda’s library of IRT customizations to expedite implementation timelines and mid-study changes
- Easily create robust and accurate eCOA questionnaires and efficiently manage licensing and localization
-