By Wilmer Barndt, Associate Director of Services Delivery
As clinical trial technology continues to evolve in an increasingly complex landscape, it’s important to understand best practices for integrating these vital resources. Successful technology integration can create a dependable ecosystem that supports every member of the team across the clinical trial workflow, ultimately getting vital treatments to patients faster.
The onset of the pandemic necessitated a sharp rise in decentralization. In this remote-enabled landscape, study teams came to rely on new technologies and the need for their integration to locate and support patients outside of the study area. These integrations ultimately worked towards a more streamlined workflow and the reduction of staff workloads.
So with these benefits, it seems as if integrating technologies would be a highly favorable choice. However, there remain barriers to adopting and integrating technologies for some trial professionals.
Barriers to Integration
In a 2021 poll by Verdict, a majority of respondents cited data authenticity, integrity, and confidentiality as a barrier to the adoption and integration of technologies. Hand in hand with this are team concerns about how they will manage data quality and ensure the system is reliable. Smaller teams may not have the expertise or staff needed to implement new technology or manage integrations.
There is also a perception that the process of adopting and integrating technologies impedes trial timelines. Therefore, study teams might believe that they don’t have time for an integrated system and, instead, elect to go the manual route, even if it requires extra work. In clinical work, data quality and integrity are critical. When manual data entry is introduced, the risk of human error goes up. Therefore, when considering the quality of the data, the benefit of integrating systems for data transfer vastly outweighs manual entry. Additionally, when data is flowing between systems, trial teams have real-time visibility of the trial data, positioning them to identify and address any issues quickly.
Large organizations may already have ecosystems made up of integrated technologies that have successfully served trial teams for years. They’re familiar with detailed aspects of how their system works. What can be a challenge for these organizations is standardizing an integrated system that serves all trials, leaving trial teams to build and rebuild individual systems for each trial.
While each of these barriers is significant, there are best practices that can streamline your clinical trial integrations. First, let’s review some of the standard systems that many trials rely on.
Clinical Trial Systems That Work Together
When organizations look at the scope and breadth of operations necessary to support a trial, it’s a good idea to consider the core clinical technologies that typically work well together. Trials typically rely on these core technologies and systems including:
- CTMS (Clinical Trial Management System)
- IRT (Interactive Response Technology)
- EDC (Electronic Data Capture)
- ERP (Enterprise Resource Planning)
- eCOA (Clinical Outcome Assessment)
Suvoda’s IRT often integrates with additional systems including supply forecasting, temperature excursion management systems used by depots, data warehouses, and central labs to transfer data and information.
Below is a diagram of a clinical technology data flow that shows what an ecosystem could look like with these individual systems working together.
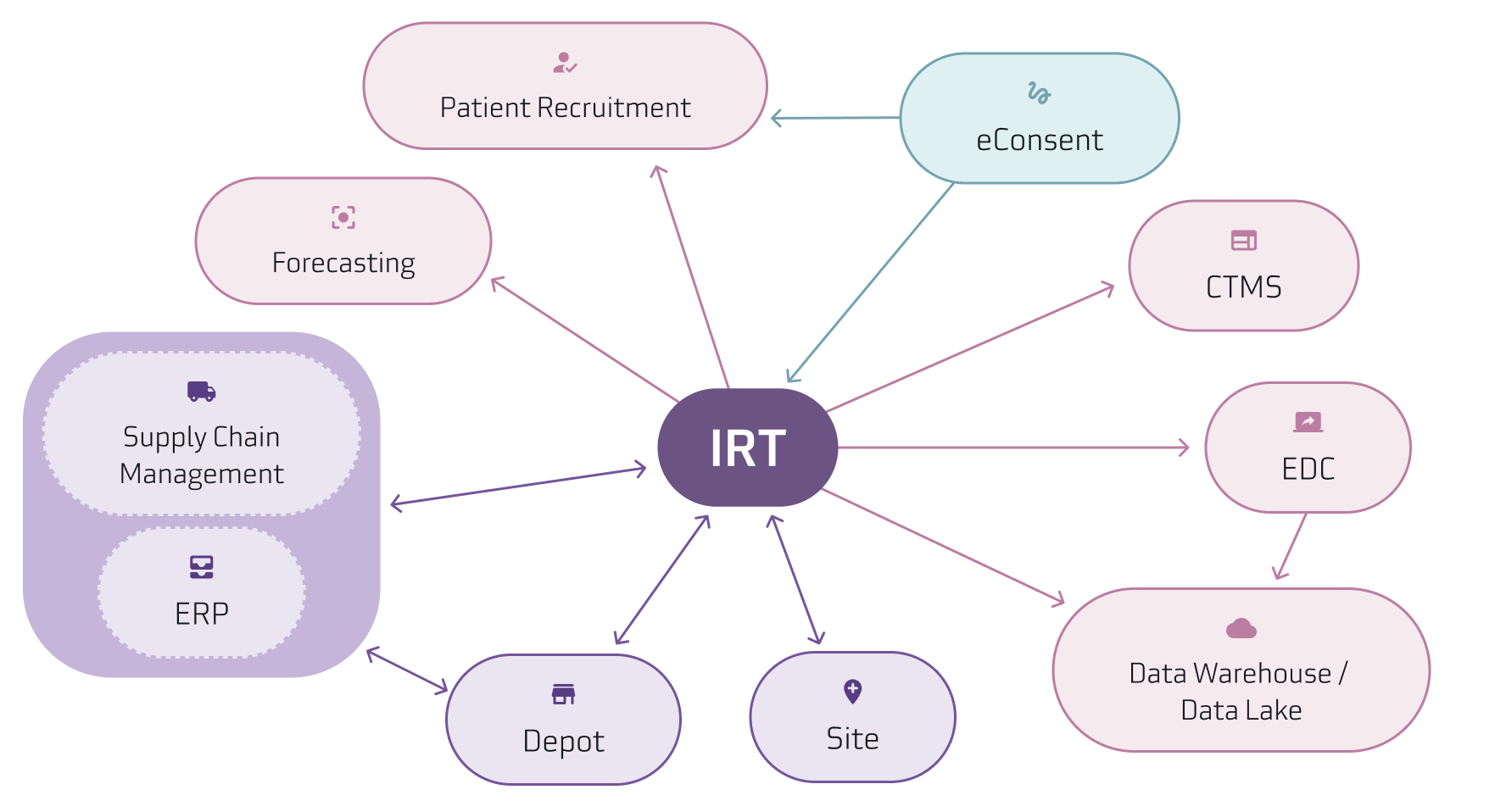
Best Practices for Technology Optimization
How you approach an integrated ecosystem is critical to how it will operate. If you take a narrow view by focusing on data transfer specifications only, your system will do this. However, it misses the opportunity for the system to solve the operational challenges inherent in every trial. If you approach it by considering how the ecosystem will function to optimize workflow and operations, you build a lasting system tailored to the nuances of your trial team's needs. Let’s consider what good practice for ensuring your system works for you is.
- Use the big picture to identify the challenges your system will solve
Whether you’re putting together a small system or replacing part of a larger one, taking a step back to consider what your ecosystem will do for you is an exercise that every trial or organization should consider. It’s a good idea to ask questions and identify gaps, such as:- What would the ideal system for this trial look like for our staff and users?
- What’s the right data set and data detail required for our protocol?
- What data points need to travel to and from systems to cut down on manual entry?
- How much efficiency do we expect to gain from the system?
- Can our organization benefit from standardizing an integrated ecosystem across trials?
Documenting your ideal system from an operational perspective gives everyone on your team, as well as your vendors, a consistent view of what the goal looks like. Ultimately, it is critical to look at how individual systems will integrate to create an ecosystem that serves the team best. - Choose vendors wisely
When looking at potential clinical technology vendors, there are many facets to consider. Be sure to investigate their methods and expertise thoroughly. Do they offer a team of dedicated integration specialists with deep clinical trial technology experience? Do they pay attention to the details that matter for trial operations? Are they using reliable, advanced technologies to manage data transfer? Will those data transfer technologies integrate well with other vendors? These are just a few of the many things to consider when evaluating a new vendor. - Consider future needs
In today’s world, data integration can’t be a one-and-done project. We have new technologies that were unknown five to ten years ago. As needs evolve and new technologies emerge, your data integration techniques should be periodically reassessed and realigned. Without consistently upgrading an ecosystem, you may face a bigger challenge with an old infrastructure that can create an unnecessary drain on trial resources. It’s important that trial organizations establish a strategy for robust, adaptable tools and frameworks that can support trials as organizational needs evolve.
Clinical trials and organizations ultimately benefit from taking a holistic approach to their technology, looking at it from all angles, and mapping a plan for today and the future. Choosing a partner with a team of integration specialists can accelerate your timeline and help eliminate risk.
Learn more about our approach to integrating technology.
Author

Wilmer Barndt
Associate Director of Services Delivery
Suvoda
