Temperature Excursion Management
Customize Criteria
- Set minimum/maximum temperature thresholds, time limits, and windows for excursion events
- Create or update criteria at any time
Streamline Review
- Register temperature data to drug kit IDs
- Automatically update status (e.g. ‘quarantined’) of drug units in real time
Enhance Visibility
- Alert study and site teams automatically when excursions occur
- Generate blinded and unblinded reports for compliance and safety
Automate your cold supply chain from depot to site
Given the nature of the novel therapeutics being developed in areas like oncology, central nervous system, and rare disease, it’s no surprise that shipping high-value, high-cost, temperature-sensitive medicines has become commonplace in our clients’ clinical trials.
That’s the focus of the Suvoda IRT Temperature Excursion Management module. It’s built for easy integration with temperature data loggers, temperature monitoring software, and depot distribution solutions. So you can provide your study and site teams with a seamless temperature management workflow.
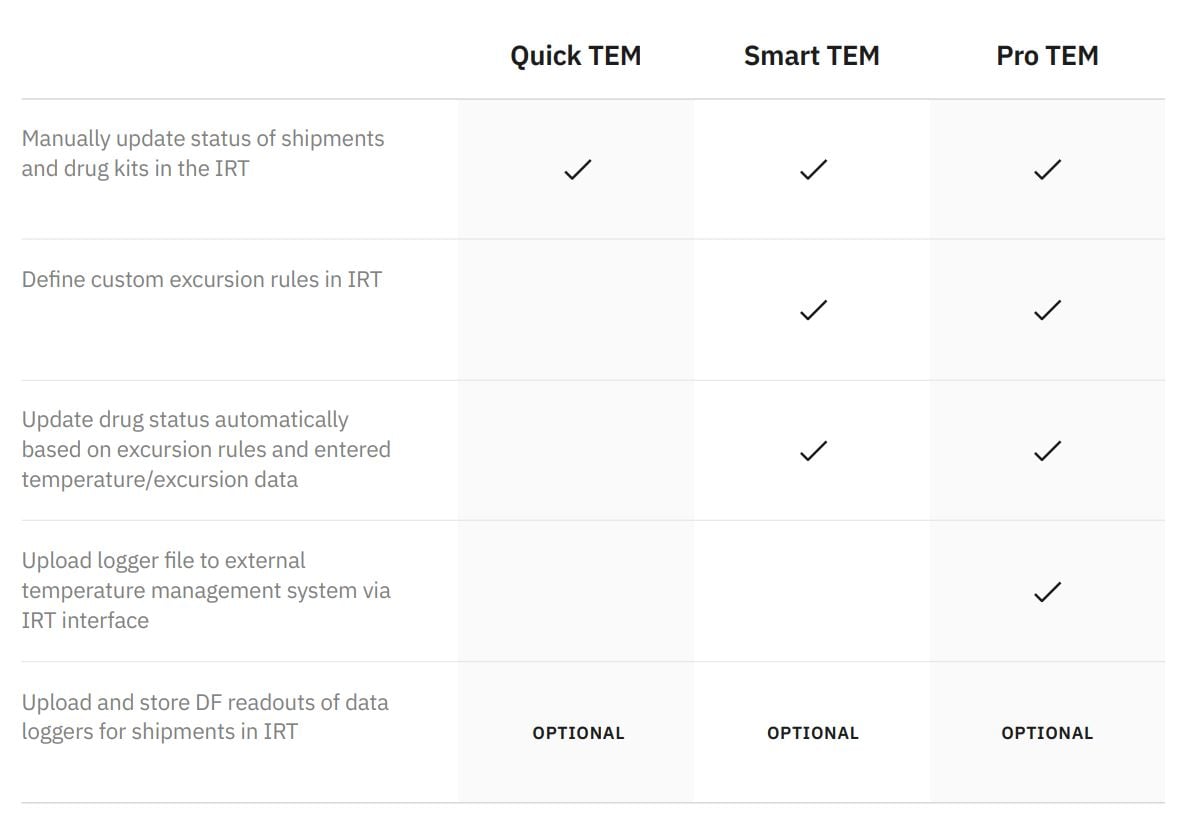
Choose the right level of management for your trial
Looking just to increase workflow efficiency and site productivity? Or to decrease the risks of manual entry errors? Or to mitigate safety and efficacy concerns? Or do you need to achieve all of the above?
We know that when it comes to IRT solutions in complex clinical trials, one size never fits all. Depending on your needs, we’ll tailor our Temperature Excursion Management functionality to give you a custom fit.
Request a demo
Ready to learn more about what Suvoda can do for your next clinical trial? Fill out the form below, and we’ll set up a meeting to walk you through our system.
REQUIRED*
